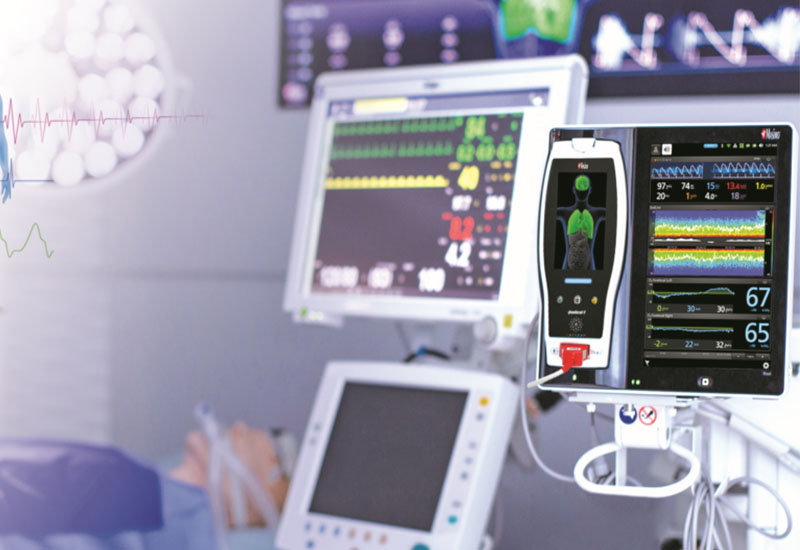
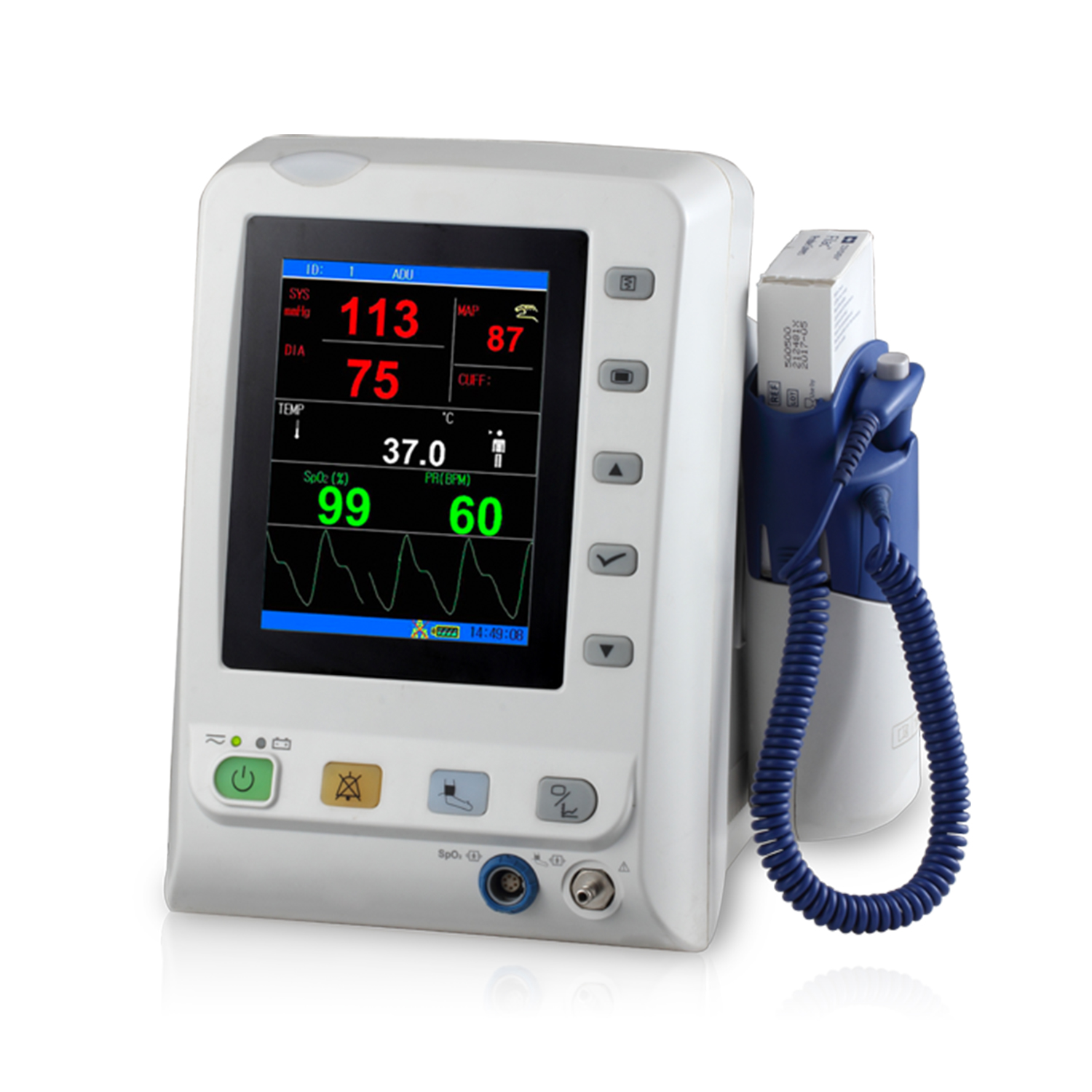
Medtronic RespArray Patient Monitor Cleared by FDA
$ 920.00
-
By A Mystery Man Writer
-
-
4.9(652)

Product Description
Medtronic plc received US FDA 510(k) clearance for its RespArray patient monitor, designed for procedural sedation and medical-surgical units.

Medtronic Respiratory Therapy

FDA Approves First-Of-Its-Kind Percept™ PC Neurostimulator with BrainSense™ Technology

Medtronic RespArray Patient Monitor Cleared by FDA
Medtronic News - Business & regional news
Medtronic RespArray Patient Monitor Cleared by FDA

Medtronic Respiratory Therapy
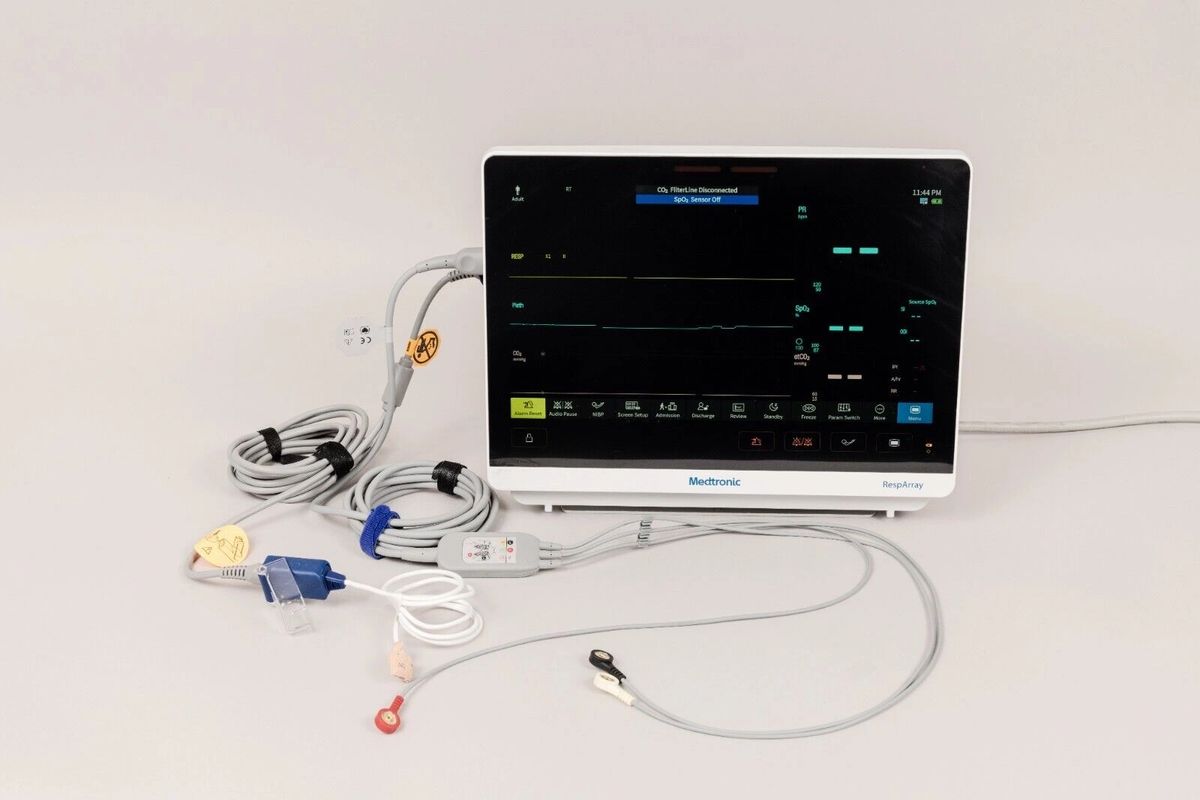
Medtronic RespArray Patient Monitor - MFG Year 2022

Understanding Capnography Waveforms
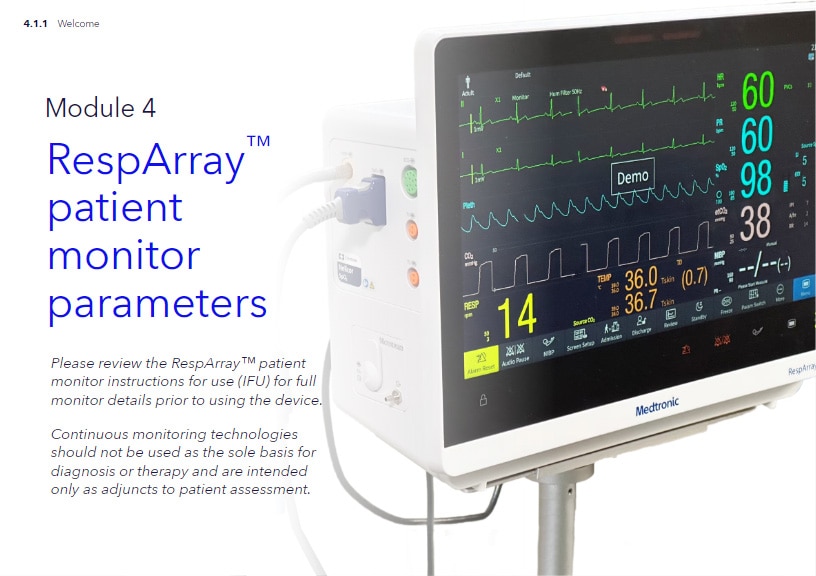
RespArray™ Patient Monitor

RespArray™ Patient Monitor