Icy Hot Heat Therapy Patch Recall Prompts FDA Warning Letter
-
By A Mystery Man Writer
-
-
4.7(497)
Product Description
Company warned for failing to notify the agency of labeling cautions added to the product after the company received more than 168 injury complaints. The maker of Icy Hot Heat Therapy patches has been issued a warning letter from the Food & Drug Administration (FDA) for failing to notify the agency of labeling cautions added to

Icy Hot Pro No-Mess Pain Relief Patches with Menthol & Camphor & Advanced Hydrogel Technology, 5 Ct.

Icy Hot Max Strength Lidocaine Pain Relief Patch (5 Count) Penetrates for Fast, Targeted Relief : Health & Household

Rael Heating Patch For Menstrual Cramps With Extra Coverage - 8ct : Target
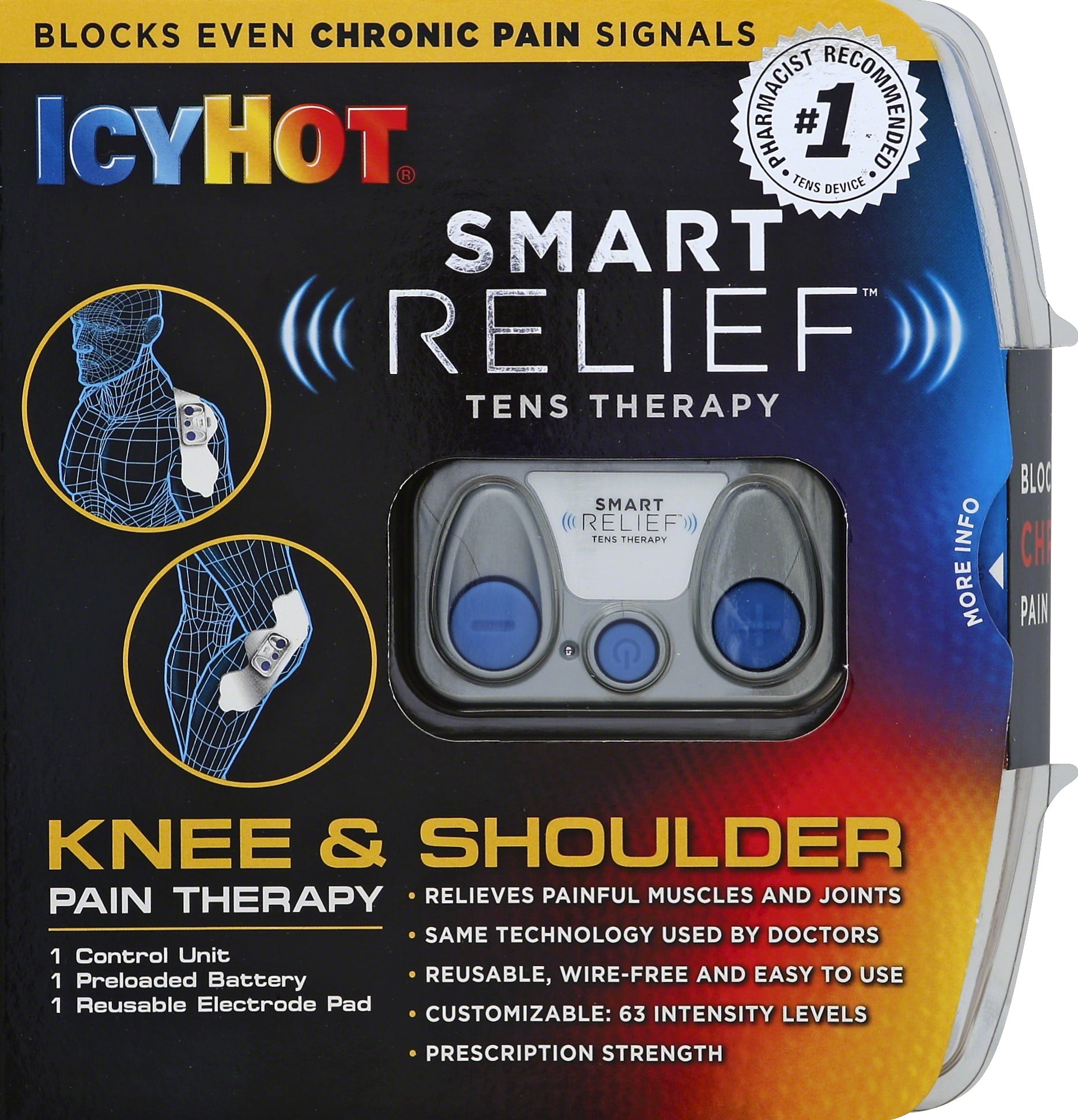
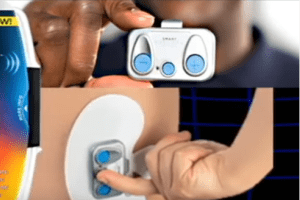
Icy Hot Smart Relief Knee and Shoulder TENS Therapy

YETI Hopper Flip 18 Soft Cooler

FDA sends warning letter over import violations and to ice cream company after Listeria found in facility

Icy Hot Max Strength No-Mess Roll-on Pain Relief Lidocaine Liquid, 2.5 fl oz - Harris Teeter

High-Throughput Methods in the Discovery and Study of Biomaterials and Materiobiology









:max_bytes(150000):strip_icc()/these-11-containers-will-help-you-achieve-an-organized-streamlined-refrigerator-0123-2000-9324962517bc4ed49e36eb7932749a20.jpg)


![Fusion Gourmet Square & Rectangle Glass Food Storage Containers with Lids [6-Pack] Meal Prep Containers, Leak proof with Airtight Locking Lids for](https://m.media-amazon.com/images/W/MEDIAX_792452-T2/images/I/81AumiANwgL._AC_UF894,1000_QL80_.jpg)


